Control Strategies: Success by Design
Quality by design (QbD) has become an important methodology in drug development. A fundamental concept of QbD is to start with the end in mind. The first step of designing a manufacturing process is to develop a quality target profile (QTP). The QTP defines the design criteria for the product. It also forms the basis for the product critical quality attributes (CQA) and control strategies.
Control Strategies in Drug Development 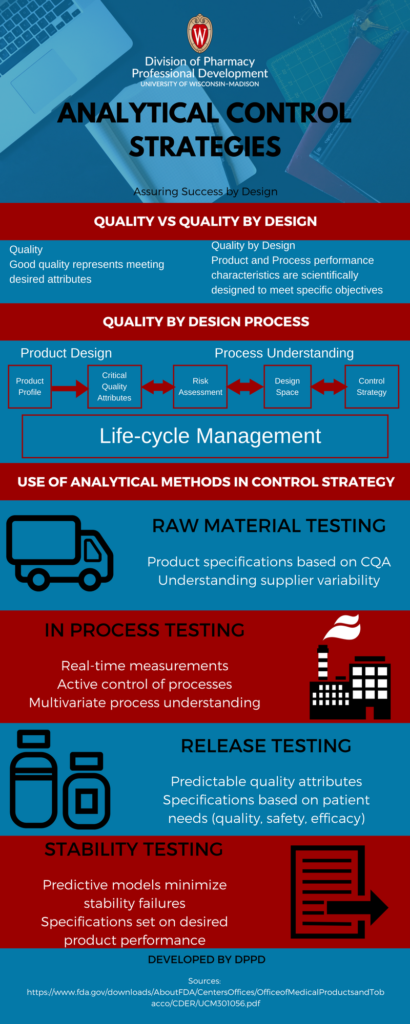
It is important to set appropriate control strategies to ensure process performance and quality. “Control Strategy” emerged in ICH Q8 and subsequent revisions. It is defined as follows:
“A planned set of controls, derived from current product and process understanding that assures process performance and product quality. The controls can include parameters and attributes related to drug substance and drug product materials and components, facility and equipment operating conditions, in-process controls, finished product specifications, and the associated methods and frequency of monitoring and control.”
This guidance was initially interpreted to be applicable to physical attributes (e.g. crystal phase, particle size) and chemical properties. ICH Q11 further clarified the concept of a control strategy as it applies to drug substance processing.
Control Strategies in Process Development
A control strategy must be part of process development. Process chemists and engineers have done so for many years. There are many approaches to developing and implementing a successful control strategy. Typical elements (as specified in Q11 but not inclusive) of a control strategy are:
- controls on material attributes
- controls implicit in the design of the manufacturing process
- in-process controls
- controls on the drug substance
Addressing Impurities
Impurities are of great importance in drug substance development. They cannot be purged downstream and must be controlled during the manufacturing process. Impurities are generally a function of physiochemical liability of the drug substance in its matrix. They can form either during processing or over its shelf life. Detecting impurities at trace levels requires highly sensitive and selective analytical methodologies.
Analytical risk can be mitigated with an Analytical Control Strategy (ACS). ACS is a planned set of controls, derived from an understanding of the requirements for fitness for purpose of the reportable value, an understanding of the analytical procedure as a process, and the management of risk. These aspects ensure the performance of the procedure and quality of the reportable value. ACS leads to consistent quality of output for the analytical procedure.
Land O’ Lakes and Control Strategies
Begin your project with the end in mind. Attend the 58th Annual Land O’Lakes conference August 6-9. This year’s theme is “Analytical Considerations for a Comprehensive Control Strategy.” You will hear industry and regulatory experts discuss topics such as:
- Small volume continuous process control strategies
- Developing clinically relevant impurity specifications
- Technology applications
- Automation of analysis
- Strategies for complex drug products
- Drug/device combo products
- Regulatory considerations
- Global control strategies
This conference will give you the tools to be a more effective scientist. You will also have an opportunity to interact with industry and regulatory leaders from around the world. Click here to learn more and register.
