Continuous Manufacturing: Batch to the Future
The standard pharmaceutical production method for over 50 years has been batch manufacturing. Batch manufacturing is a multi-step, lengthy process that involves large-scale equipment. The pharmaceutical industry has been looking for ways to become more efficient. Recent advances in manufacturing technology are creating a faster more efficient process known as continuous manufacturing.
Transitioning to continuous manufacturing in a tightly regulated environment is not easy. It requires regulatory approval from the FDA. Continuous manufacturing has many benefits that can improve drug quality. It also has the ability to prevent issues like drug shortages and recalls. To improve drug manufacturing, the FDA is making efforts to support continuous manufacturing.
Batch Manufacturing: A Myriad of Shortcomings
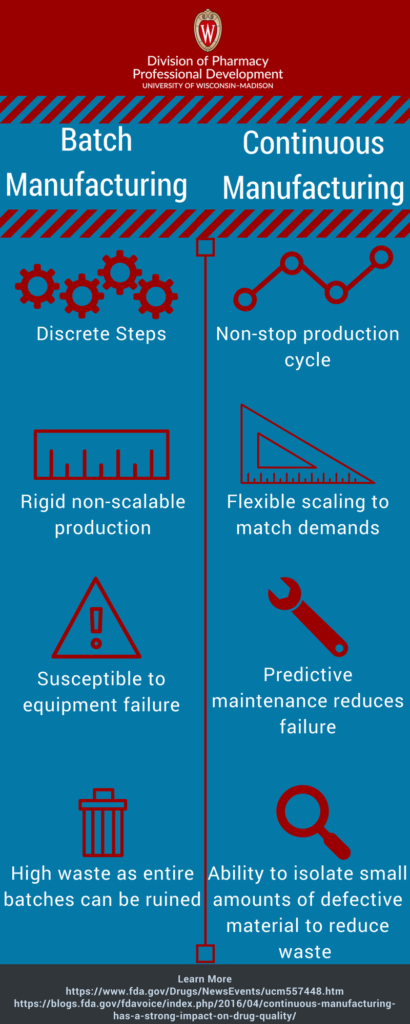 Batch manufacturing consists of many discrete steps. Production stops between each step to test samples for quality assurance. The time between steps can be hours to weeks. In some cases, the material moves through multiple facilities.
Batch manufacturing consists of many discrete steps. Production stops between each step to test samples for quality assurance. The time between steps can be hours to weeks. In some cases, the material moves through multiple facilities.
The large gaps in time put environmentally sensitive ingredients at risk for degradation. This method also lacks the flexibility to scale production to meet increased demand.
Continuous Manufacturing: A Better Process
Continuous manufacturing shortens the production process and reduces the likelihood of human error. The assembly line integrates all necessary components. Pharmaceuticals to move nonstop within the same facility with no hold times. It is also more flexible and can respond to market changes faster than the batch method. The continuous manufacturing process can run for a longer period of time to meet increased demand.
Enhanced Drug Quality Outcomes
The quality control standards are the same for both types of manufacturing. However, continuous manufacturing uses automated monitoring that allows frequent analyses. Monitoring can also detect the inevitable wear and tear issues before failures occur. Life expectancy of equipment is predicted in real time leading better proactive maintenance. This approach prevents failures from occurring during manufacturing. Failures occurring during the batch manufacturing process can ruin entire batches.
Continuous manufacturing allows more flexible tracking in the event of a product failure. In batch manufacturing the size of the equipment determines the batch size. Continuous manufacturing has flexible options for batch size. Batch size can be determined by time stamp, the amount produced, or raw material input. In the event of process failure, it is possible to isolate a smaller account of defective material. This leads to less waste and lower probability of shortages.
Drug Manufacturing for the Future
Continuous manufacturing is not a new concept; the chemical and petrochemical industries are already reaping its benefits. However, the pharmaceutical industry has many hurdles for implementation. Startup costs are already high and continuous manufacturing may add additional costs for revamping the industry infrastructure. The biggest hurdle, however, is regulatory approval.
Faster and cheaper production means nothing if there is regulatory uncertainty. Delays in product approval due to new manufacturing technology can be the difference between success and failure.
The FDA is providing resources and information to facilitate the transition. The agency is engaging the pharmaceutical industry to proactively address regulatory and scientific issues.
June Land O’ Lakes Conference
Good speakers and knowledgeable presentations. Very interactive and time to network w/ speakers and other participants. – June Land O’ Lakes participant
Land O’Lakes conferences are recognized worldwide for their contribution to pharmaceutical sciences. The 60th June Land O’Lakes Conference focuses on formulation development and drug delivery. Along with sound science, the informal and interactive environment fosters learning and exchange of ideas.
This year’s conference theme is:
“Technical and Regulatory Considerations for Development and Commercialization of Drug Substance and Drug Product Continuous Manufacturing.”
Be part of the continuous manufacturing revolution by attending this year’s meeting. You will learn first-hand about continuous manufacturing issues like:
- API
- Formulation development
- Manufacturing process control
- Regulatory compliance
and much more
Continuous manufacturing is here to stay in the pharmaceutical industry. Do not miss this chance to shape the current and future state of formulation and development.
The topics were focused on Pharmaceutical Development and everything covered was directly applicable to my work. – June Land O’ Lakes participant
When: June 4th to 7th, 2018
Where: Fluno Center, University of Wisconsin-Madison campus
Learn More>>
References
https://blogs.fda.gov/fdavoice/index.php/2016/04/continuous-manufacturing-has-a-strong-impact-on-drug-quality/
https://www.fda.gov/Drugs/NewsEvents/ucm557448.htm
