Biomarkers: Growing Potential
A biomarker is a defined characteristic that is a measurable indicator of a biological process. Biomarkers measure normal or pathogenic processes. They may also measure pharmacologic responses to therapeutic intervention. Biomarkers can have molecular, histologic, radiographic, or physiological characteristics. However, they do not assess how an individual feels, functions, or survives.
Biomarkers have the potential to reduce time and costs. This makes them an important aspect of drug development. They have roles spanning from early development to clinical trials. Some examples of what biomarkers can do are:
- Monitor safety of a therapy
- Determine if a treatment is having a desired effect on the body
- Predict patients who might respond better to a drug
- Predict drug safety and efficacy
Seven Types of Biomarkers
There are seven biomarker categories defined by BEST*. A biomarker may fall under multiple categories depending upon the application. 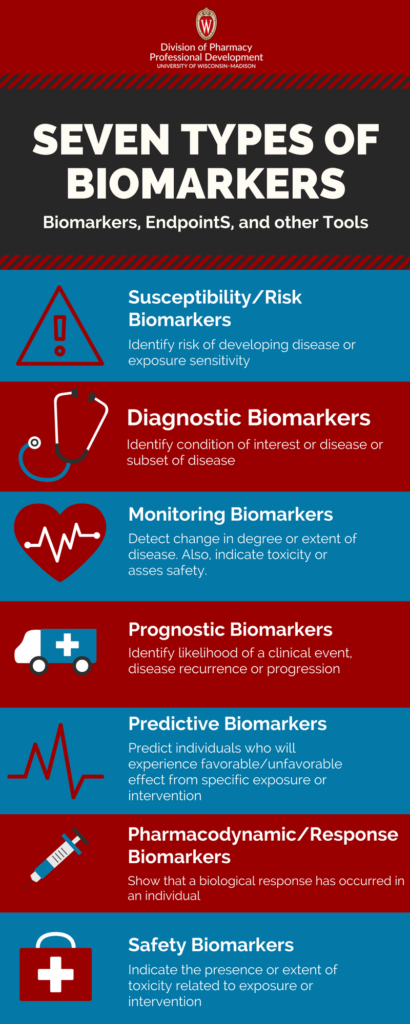
- Susceptibility/Risk biomarkers state the risk of developing a disease or exposure sensitivity. E.g. BRCAI/2 mutations
- Diagnostic biomarkers identify individuals with the condition of interest or disease or subset of disease. e.g. Sweat chloride can be used as a biomarker for cystic fibrosis (CF)
- Monitoring biomarkers detect a change in the degree or extent of disease. They can also indicate toxicity or assess safety. e.g. Hepatitis C virus ribonucleic acid (HCV-RNA) used to track patients with Hepatitis C to evaluate disease status or burden
- Prognostic biomarkers identify the likelihood of a clinical event, disease recurrence or progression. e.g. BRCAI/2 mutations used to test women with breast cancer to assess the likelihood of recurrence.
- Predictive biomarkers identify individuals who are likely to experience a favorable/unfavorable effect from a specific exposure or intervention. e.g. Epidermal Growth Factor Receptor (EGFR) mutations are a predictive biomarker for evaluation of non-small cell lung cancer patients to select patients for anti-EGFR therapy.
- Pharmacodynamic/response biomarkers show that a biological response has occurred in an individual. e.g. Circulating CD20-positive B lymphocytes is a biomarker for evaluating patients with lupus erythematosus to assess response to B-lymphocyte stimulator inhibitor.
- Safety biomarkers indicate the presence or extent of toxicity related to exposure or intervention. e.g. Hepatic aminotransferases are evaluated to for potential hepatoxicity.
Biomarkers in Drug Development
Biomarkers have the potential to shorten clinical trial times with surrogate endpoints. A surrogate endpoint is “an endpoint used in clinical trials as a substitute for a direct measure of how a patient feels, functions, or survives.” It does not measure the clinical benefit of primary interest in and of itself. It uses epidemiologic, therapeutic, pathophysiologic, or other scientific measures to predict clinical benefit or harm.
Biomarkers used as outcomes can speed up product development in certain disease areas. A conventional approach might measure the performance of novel therapies using clinical outcomes like mortality or disease progression. Accruing enough data for clinical endpoints can take many years. Using a biomarker-driven approach allows quick prediction of drug efficacy. This approach can shave years off product development.
While biomarkers are a powerful tool, there are barriers to their use in drug development. Some of these barriers faced are
- Lack of scientific evidence
- Lack of reproducibility of results
- Limited resources for biomarker development
Land O’Lakes and the future of Biomarkers
The Center for Drug Evaluation and Research (CDER) at the FDA is making efforts to reduce barriers. CDER is encouraging collaborative efforts and data sharing among biomarker developers.
The 19th Annual Land O’Lakes Bioanalytical Conference will be held from July 9-12 in Madison, WI. This year’s theme is “The Next Generation of Biomarker and Bioanalytical Research“. Presentations will focus on emerging therapeutic paradigms and the shifting regulatory landscape. It is an excellent opportunity to connect with biomarker experts from around the world.
Land O’Lakes is a premier conference series for pharmaceutical scientists. The Bioanalytical conference is renowned for its high caliber presentations and speakers. This conference fosters interaction and exchange of ideas in a friendly collegial atmosphere.
Click here to learn more and register
Reference: Biomarkers, EndpointS, and other Tools https://www.ncbi.nlm.nih.gov/books/NBK326791/
